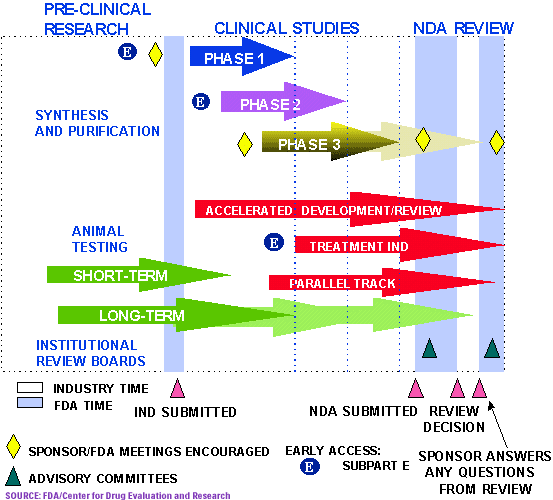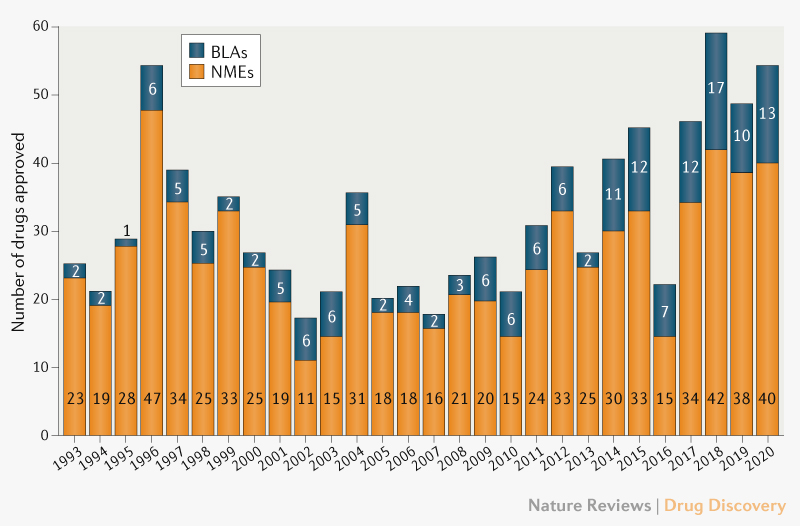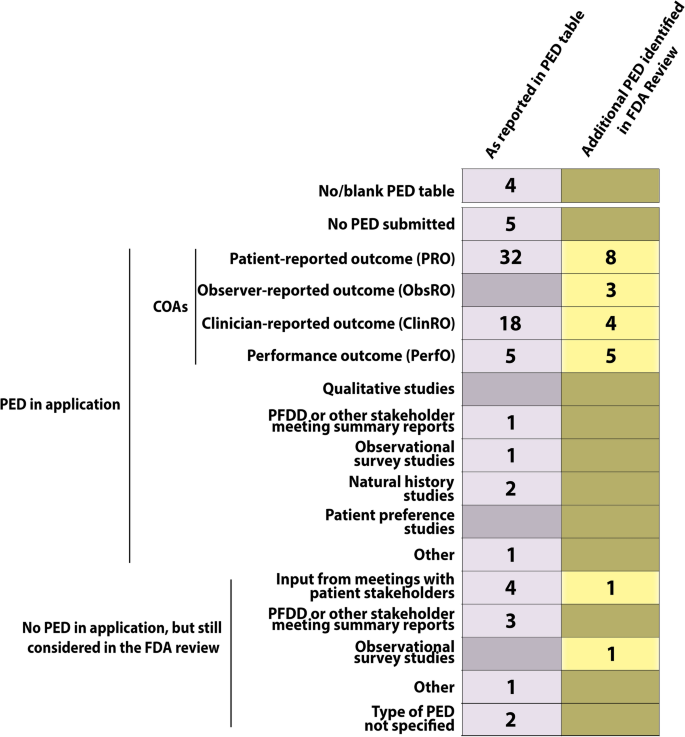
New Drug and Biologics Approvals in 2019: A Systematic Analysis of Patient Experience Data in FDA Drug Approval Packages and Product Labels | SpringerLink
U.S. FDA Approval Summary: Nivolumab for Treatment of Unresectable or Metastatic Melanoma Following Progression on Ipilimumab
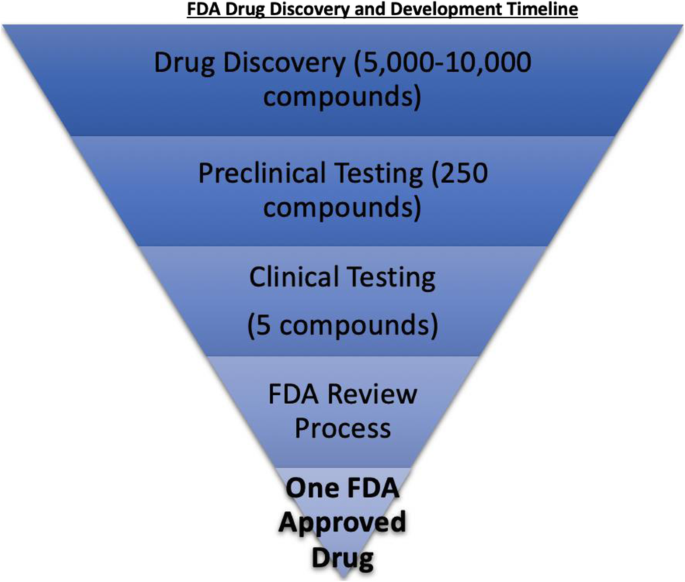
FDA efficiency for approval process of COVID-19 therapeutics | Infectious Agents and Cancer | Full Text

Fillable Online fda Product Approval Information Summary Basis of Approval OCTAGAM 5% - fda Fax Email Print - pdfFiller

Drugs, Devices, and the FDA: Part 2: An Overview of Approval Processes: FDA Approval of Medical Devices - ScienceDirect
Comparison of FDA accelerated vs regular pathway approvals for lung cancer treatments between 2006 and 2018 | PLOS ONE

Frontiers | A Regulatory Risk-Based Approach to ATMP/CGT Development: Integrating Scientific Challenges With Current Regulatory Expectations

Fillable Online fda SUMMARY BASIS FOR APPROVAL PLA - Food and Drug ... - fda Fax Email Print - pdfFiller
Publication of Clinical Trials Supporting Successful New Drug Applications: A Literature Analysis | PLOS Medicine